How to excel in CBSE Class XII?
Preparation of CBSE Class XII Board Biology
There is always big question in mind of students appearing in Class XII i.e. How to excel in CBSE Class XII? For medical career aspirants it is a no-brainer that Biology is the most important subject in Class 11th and 12th. This article will focus on things to keep in mind during preparation with some insight into scope of biology in biotechnological fields.
Syllabus
The biology paper is for 70 marks (theory) in CBSE and the following is the weightage of the individual chapters. Reproduction (14 marks – 20%), Genetics and Evolution (18 marks – 25%), Biology and Human Welfare (14 marks – 20%), Biotechnology and its Applications (10 marks – 15%) and Ecology and Environment (14 marks – 20%). In AIPMT, 90 questions are asked in Biology (Botany + Zoology) with each question 4 marks.
Notes and Diagrams
Preparing notes at the time of teaching would become very useful in a conceptually dependent subject like Biology. Not only does it help in the process of remembering the terms while writing, it also helps in the student’s last minute preparation strategies. A question might have a huge theoretical answer but this can be reduced to a simple explanative answer by drawing the necessary diagrams (case in point – DNA structure, cell biology etc.).
Chapterwise
Chapters like Genetics and Biotechnology need to be understood well to make them easy. They go hand in hand and hence should be prepared together. The chapters that carry 14 marks each (reproduction, ecology and human welfare) are broad which contain a lot of detailed sub topics and hence should be prepared first.
Cramming?
Do not try to cram the subject as it will make you miss out on important points for exam. Instead, prepare on a consistent basis of at least 2 to 3 weeks prior to the exam.
Productive Breaks
If you know your concentration is going to wander of course it is better to take breaks in between topics. During these breaks try to recollect what was studied and/or try practicing diagrams and be resourcefully productive.
Flashcards
One strong advice from alumni would be to create “flashcards” for remembering definitions and labeling parts in diagrams. It is better to take notes in class in points/bullets as the order of answers fetches you more marks. It is important to revise the course at least 3 times before an examination. For lengthy biological names use mnemonics. Break up the names in a way that will aid in remembering them.
Past year papers
It is a good practice to sit and write the past year exam and sample papers with a timer set to simulate the exam day. This will help in creating awareness of time management and will help relieve stress caused due to availability of less time in answering theoretical long answer type questions.
Practical exam
The 30 marks of Practical exam can be easily scored if one pays attention in the lab classes. Even though you will be assigned lab partners, it is important to experience firsthand as the final examination will only be that way.
For more info please check the links below
Class 12+ – Syllabus, Exam Pattern and Previous Papers with solutionsCentral Board of Secondary Education – CBSE Exam
Topprs
How to excel in CBSE Class XII Chemistry
Along with important topics, it is always important to understand the type, marking scheme and answering technique for the questions.
I will try to put together all of it in the following answer :
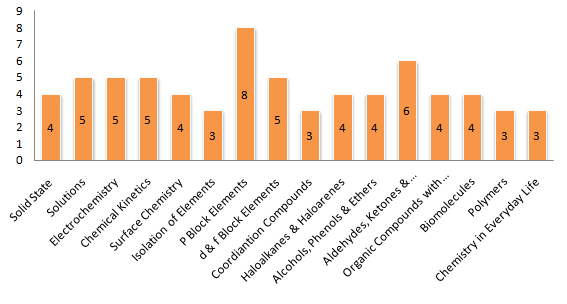
Let’s have a look at some stats (as per 2018 chemistry paper ) to understand the chemistry paper marks distribution
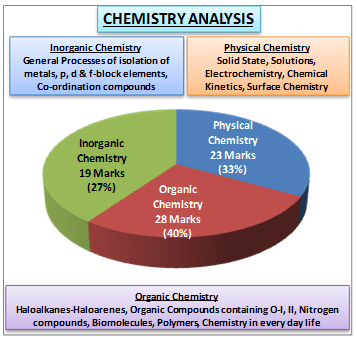
Overall Distribution :
Chapter 1: Solid State
Important Topics:
1. Number of octahedral voids = Number of atoms and Number of tetrahedral voids = Twice the number of atoms
2. For unit cell dimension the important formula is M = dNaa3/ z
3. Imperfections in solids:
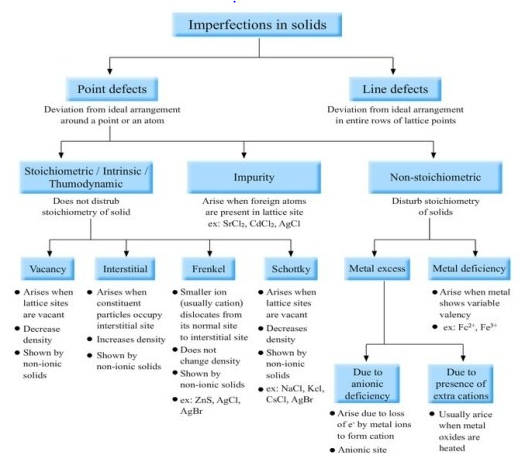
Chapter 2: Solutions
Important Topics:
1. Raoult’s law
2. Dalton’s law of partial pressure
3. Henry’s law
4. Mass % = (Mass of component in solution/ Total mass of the solution) ✕ 100
5. Volume % = (Volume of component in solution/ Total volume of the solution) ✕ 100
6. ppm = (No. of parts of the component/ Total no. of parts of all components of solution) ✕100
7. Mole fraction = No. of moles of component/ Total no. of moles of all components
8. Molarity = (Moles of solute/ volume of solution in litres)
9. Molality = Moles of solute/ Mass of solvent in kg
10. Relative Lowering of vapour pressure
11. Elevation of boiling point
12. Depression of freezing point
13. Osmosis and Osmotic Pressure
14. Van’t Hoff Factor
Chapter 3: Electrochemistry
Important Topics:
1. Type of cells:
a) Electrochemical cell / Galvanic cell
b) Electrolytic cell
2. Electrode potential
3. Standard electrode potential
4. Anode
5. Cathode
6. Cell potential
7. Cell electromotive force (emf)
8. SHE (Standard Hydrogen Electrode)
9. Nernst equation
10. The relation between cell potential and Gibbs energy:
11. Relation between equilibrium constant and Gibbs energy
12. Resistance(R)
13. Resistivity
14. Specific conductance/Conductivity
15. Molar conductivity
16. Molar Conductivity for Strong Electrolytes
17. Molar Conductivity for Weak Electrolytes
18. Kohlrausch law of independent migration of ions
19. Faraday’s law of electrolysis
20. Types of electrodes
a) Inert
b) Active
21. Batteries:
a) Primary
b) Secondary
22. Corrosion
23. Hydrogen Economy
Chapter 4 – Chemical Kinetics
Important Topics:
1. First Order Reaction Kinetics
2. Arrhenius Equation
3. Order and Molecularity
Chapter 5: Surface Chemistry
Important Topics:
1. The distinction between adsorption and absorption
2. Types of Adsorption
3. Freundlich adsorption isotherm
a) Adsorption from Solution Phase
4. Colloids
5. Emulsions
INORGANIC CHEMISTRY
Chapter 6: General Principles and Processes of Isolations of Elements
Important Topics:
1. Metallurgy
2. Ellingham Diagram
3. Mond process for refining nickel
4. Van Arkel Method for refining zirconium or titanium
Chapter 7: The p-Block Elements
Important Topics:
1. Inert pair effect
2. Disproportionation
3. Non-metallic hydrides
4. Colour of halogen compounds
5. Non-metallic halides
6. Catenation
7. Interhalogen compounds
8. Structures of Oxoacids: Phosphorus and Sulphur
9. Structures of Fluoride: Sulphur, xenon, bromine
10. Basicity of group 15 elements
11. Structures of PCl5, H2SO3, H2SO4, H2S2O8, H2S2O7, HOCl, HClO2, HClO34, N2<O5, XeOF4
Chapter 8: The d- and f-block Elements
Important Topics:
1. d-Block elements
2. f-Block elements
3. Actinoid
4. Lanthanoid Contraction
5. Actinoid Contraction
Chapter 9: Coordination Compounds
Important Topics:
1. Coordination Compounds
2. Coordination Entity
3. Central Atom or Ion
4. Ligands
5. Coordination Number
6. Coordination Sphere
7. Coordination Polyhedron
8. Oxidation Number of Central Atom
9. Homoleptic complexes
10. Heteroleptic complexes
11. Werner’s theory
12. Valence bond theory
13. Crystal-field theory
ORGANIC CHEMISTRY
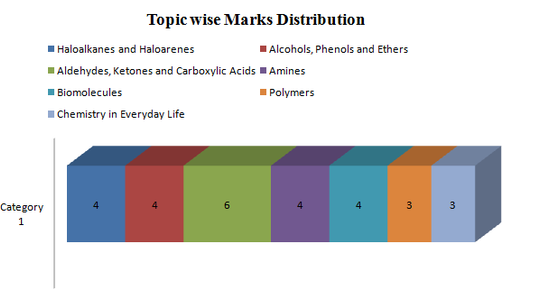
(Chapter 10 to Chapter 16)
Important Topics:
1. IUPAC Nomenclature questions
2. Name reactions: Important name reactions which have been asked previously are Sandmeyer reaction, Williamson synthesis, Riemer-Tiemann reaction, Kolbe’s reaction, Aldol Condensation, Cannizzaro Reaction, Clemmensen Reduction reaction, Hoffmann Bromamide reaction, Coupling reaction. So,we advise you to revise these reactions.
3. Distinction test: The distinction tests are usually asked between:
4. Aliphatic and aromatic compounds
5. Compounds having two different functional groups
6. Compounds having same functional group but different arrangement of atoms (e.g., 1°, 2°, 3°)
Steps for attempting these questions:
Step – I: See how many marks are allotted to the question. Remember, 1 mark is for 1 test.
Step – II: Write the structural formulae of both the compounds.
Step – III: See where the two structures differ in.
Step – IV: Recall the reactions which you have studied.
Step – V: Apply those reactions in the compounds keeping in mind the skeletal structure they differ in.
7. Conversions: Conversion based questions are surely going to come in exams. Remember there can be multiple steps to reach the final product but the shortest and feasible steps have to be written in the answer-sheet.
(Please note CBSE has yet not asked any conversion which consist of more than 3-steps.)
Steps for attempting these questions:
Step – I: Read the question very carefully.
Step – II: Write the starting compound on the left hand side and the final compound on the right hand side.
Step – III: See where do the two structure differ in.
(They mostly differ either in functional groups, number of carbon atoms or both)
Step – IV: Recall the reactions which you have studied.
Step – V: Apply those reactions in initial compound so as to reach to the final compound.
TIPS:
a) Focussing on NCERT questions: The NCERT Part – II comprises of organic chemistry and contributes to 28 marks in the examination.
b) An analysis of previous years’ question papers depicts that many questions are asked as it is from the NCERT textbooks.
Physics Preparation
Physics is a very important subject, not only for board exams, but also for the competitive exams. You should regularly read and practice physics, from your school textbook (Eg: NCERT). You need to make a good balance between numerical problems and definitions or theoretical problems.
Tips to score in physics :
- Go through NCERT book. It is the most important book for Physics board exam of class 12. All topics in the syllabus for the exam are covered in the NCERT book.
Practice each and every worked-out example, graph and diagram, especially-
a) Ray diagram for Astronomical Telescope, Compound Microscope and Reflecting Telescope.
b) Diagrams for few devices like cyclotron and potentiometer to name a few.
- Refer a supplementary book like Haliday-Resnick-Walker only if you do not understand a certain concept from the NCERT book and to practice more numerical problems.
- Make a complete list of derivations, formulae and experiments in your syllabus and keep that list handy.
- While solving a derivation, try and comprehend the logic behind the derivations.
- If you do not like the numerical part, start early! Get used to the numerical part. Solve each and every numerical in the NCERT book (both, solved and unsolved).
- Those who have not good numerical skills need to prepare Semiconductor, Atom and Nuclei, Dual nature of radiation, Communication System and EM Waveswhich carry 25 marks. Focus upon Optics which also carry 14 marks. Current and Electricity also contains 7 marks.
- Use Reference books for reference purpose only. Remember, your textbook is NCERT.
- Do Analysis of the last 10-year Question Paper.
- Solve model sample papers.
- Be positive. It’s a boost for concentration and better retention.
- Time management is a must; take out time for re-creation as well as alongside preparation.
- During the exam, try to write your descriptive answers in points and give pictorial or graphical illustrations wherever possible. It enhances visibility.
Some of the important topics in Physics, for class 12th CBSE exams are (As per weightage):
- Optics (Both Wave and Ray optics) – 14 marks
- Electrostatics – 08 marks
- Magnetic effect of current and magnetism – 08 marks
- EMI and AC – 08 marks
- Current electricity – 07 marks
- Electronic Devices – 07 marks
- Atoms and Nuclei – 06 marks
- Communication systems – 05 marks
- Dual nature of radiation and matter – 04 marks
- EM Waves – 03 marks
To know about our NEET and JEE preparatory Courses
https://whizdomedu.com/